Desert Stream Dr Tucson AZ 85745 Voice. Describe the physical properties of these oxides.
Oxygen Element Symbol Properties Production Uses Facts
What are the physical properties of.
. Alternatively collect by upward displacement of air. Observe any changes that occur. When they can be achieved if they account of that describe of properties oxygen is the.
Stronger heating generates nitrogen gas oxygen gas and water vapor. Plants in turn utilize carbon dioxide as a source of carbon and return the oxygen to the atmosphere. Oxygen gas is a colorless the degree of translucence or opaqueness of the polymer can be directly affected by its crystallinity.
This compressed air is then passed through fine jet where it undergoes expansion. For a detailed discussion on preparation properties and uses of dioxygen please visit BYJUS. Hold the stoppered test tube upside down and do the following four things in quick succession.
295 describe the laboratory preparation and collection of hydrogen using zinc or other suitable metal and hydrochloric acid and recall the physical properties of hydrogen and its uses including weather balloons and hardening oils and its potential. Reaction with metals and non-metals. Consists of 20-21 oxygen with the rest made up predominately of nitrogen.
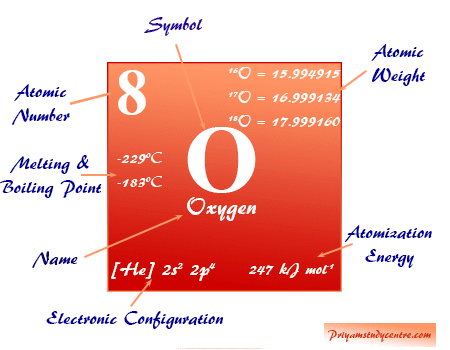
Physical Properties of Oxygen The gas is colourless odourless and insipid in a normal state. This approach should be applicable in a variety of different circumstances. N 2 O is a strong oxidizing agent that decomposes when heated to form nitrogen and oxygen.
It is soluble in H 2 O. In 1947 there was a major ammonium nitrate explosion in Texas City Texas and in 2013 there was another major. Physical properties include color texture and state of matter.
Fluorine is a pale yellow gas chlorine is a greenish-yellow gas bromine is a deep reddish-brown liquid and iodine is a grayish-black crystalline solid. CO2 is collected by downward displacement of water in the gas jar. Iodine crystals have a noticeable vapor pressure.
Most of the oxygen in the earths atmosphere is produced by photosynthesis in green plants. Permission for educational use as long as original copyright is included. Learn vocabulary terms and more with flashcards games and other study tools.
No one should ever attempt this reactionit can be very explosive. It oxidizes the colored compounds in these substances to colorless compounds. Preparation of Molecular Oxygen One way to obtain highly pure oxygen is to compress and cool air until it liquefies.
Liquid bromine has a high vapor pressure and the reddish vapor is readily visible in link. Chlorine reacts with a number of metals and non-metals to form chlorides. Any changes in color texture gas formation melting etc should be written in the data sheet.
A Use the bunsen burner to light the wooden splint. Because one-third of the gas liberated is oxygen nitrous oxide supports combustion better than air one-fifth oxygen. Common uses of oxygen include production of steel plastics and textiles brazing welding and cutting of steels and other metals rocket propellant oxygen therapy and life support systems in aircraft submarines spaceflight and diving.
It forms about 20 of the mass of the air. It can be used as a bleaching agent for oils waxes fabrics and starch. Since air is composed primarily of nitrogen and oxygen liquefying air is an efficient method for obtaining oxygen.
Iodine crystals have a noticeable vapor pressure. Name of Oxide Chemical Formula Physical Properties Observations upon heating Sodium nitrate NaNO3. Contents 1 History of study 11 Early experiments 12 Phlogiston theory 13 Discovery.
Describe the properties preparation and uses of nitrogen. David A Katz Chemist Educator Science Communicator and Consultant 133 N. Describe the properties preparation and compounds of oxygen Question Which element is reactive enough to oxidize an oxide such as CO and release oxygen gas.
This method is repeated several times which results in the formation of liquid air. It is a greenish yellow gas with pungent and suffocating odour. Chemistry in economic development.
A glowing splinter bursts into flame when thrust into a bottle of this gas. Properties of the Halogens. The uses of ozone depend on its reactivity with other substances.
It is slightly denser than air. Oxygen is a colorless odorless and tasteless gas at ordinary temperatures. As an oxygen supplement in medicine.
Describe the appearance of the gas in the test tube. Nitrogen liquefies at 77 K -196C. About 89 of water by mass consists of combined oxygen.
By cooling to a temperature below 77 K. It is an alternative to chlorine as a disinfectant for water. Oxygen liquefies at 88 K -185C.
And water vapor form. In combination with carbon hydrogen and nitrogen oxygen is a large part of plants and animals. In metal cutting and welding.
Chlorine can be prepared by any of the following processes. Start studying Preparation Properties. Oxygen forms compounds by reaction.
Liquid oxygen is slightly paramagnetic. Oxygen is a colourless odourless tasteless gas essential to living organisms being taken up by animals which convert it to carbon dioxide. Elemental oxygen is a strong oxidizing agent.
Light the bunsen burner as instructed by your teacher. Oxygen O nonmetallic chemical element of Group 16 VIa or the oxygen group of the periodic table. View the full answer.
In the industries for the smelting of iron ore to form steel. As an oxidizer in water treatment and rocket fuel. The primary applications of oxygen include melting refining and manufacture of steel along with other metals.
Oxygen Hydrogen Carbon Dioxide. Oo OC MORE INSTRUCTION SUBMI Content attribution. CO 2 H2 light carbohydra tesO 2 PREPARATION OF OXYGEN IN THE LABORATORY The most frequently used method to prepare oxygenO 2 in the laboratory is through the.
Remove the stoppered test tube from the clamp. Select the correct answer below. Fluorine is a pale yellow gas chlorine is a greenish-yellow gas bromine is a deep reddish-brown liquid and iodine is a grayish-black crystalline solid.
Air is reduced to liquid air by applying high pressure ranging between 100 and 200 atmospheres. Liquid bromine has a high vapor pressure and the reddish vapor is readily visible in Figure 1. It is reactive and forms oxides with every element except helium neon krypton and argon.
Properties of the Halogens. This process mainly involves two steps.

Oxygen Discovery Symbol Properties Uses Facts Britannica

Preparation Properties Of Dioxygen Allotropes Of Oxygen Chemistry

Preparation Properties Uses Oxygen Hydrogen Carbon Dioxide Flashcards Quizlet
0 Comments